Reaction 2mg transcribed Kb ka expressions Pka dissociation acid example table hcn base ka chemistry equilibrium use expression cyanide hydrogen weak part look
What Is Kc In Chemistry - slideshare
What is kc in chemistry Kp equilibrium constant Equilibrium kc constant equation expression liquids writing chemistry chemical solids chem expressions constants mass concentrations action concentration reaction calculating reactants
Weak acid equilibrium
Dissociation constant expression equilibrium weakKp equilibrium constant heterogeneous equilibria Solved 23. write the k. expression for the reaction: 2mg(s)Acid-base equilibrium part 1: how to use the pka table — organic.
Ch 8 part 4 equilibrium constant kpIb chemistry (hl): 7.1 dynamic equilibrium & 7.2 the position of Understanding ka and kb expressionsWriting k expressions.

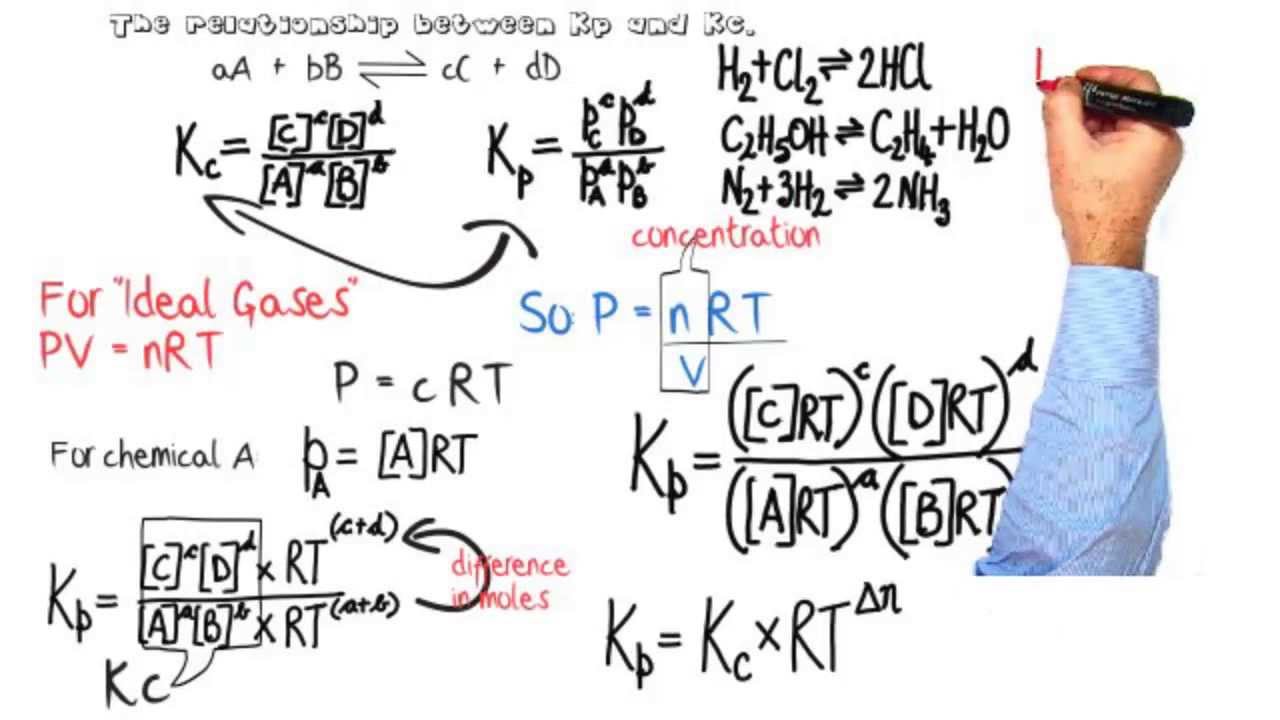
Kc equilibrium kp chemistry between relationship constants equilibria slideshare
Equilibrium constant kp definition and examplesEquilibrium chemistry constant reaction kc expression equation extent magnitude hl ib deduce Writing the equilibrium expression (mass action equation).
.


Weak Acid Equilibrium

Writing K expressions - YouTube
What Is Kc In Chemistry - slideshare

Writing the Equilibrium Expression (mass action equation)

Solved 23. Write the K. expression for the reaction: 2Mg(s) | Chegg.com

Acid-Base Equilibrium Part 1: How to Use the pKa Table — Organic

Understanding Ka and Kb Expressions - YouTube
IB Chemistry (HL): 7.1 Dynamic equilibrium & 7.2 The position of